Innovative Capabilities in Norwalk: Precision Medical Machining And Endoscope Expertise
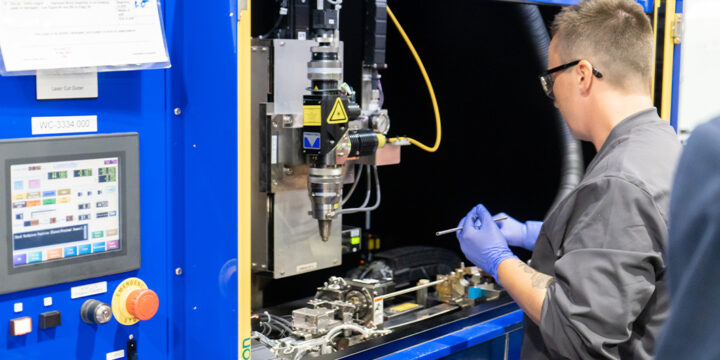
By Amy Jeary The Nissha Medical Technologies facility in Norwalk, Ohio has been providing precision medical machining services to medical OEMs for over six decades. Prior to becoming part of NMT in 2020, the Norwalk facility has maintained a long-standing reputation for excellence in machining and assembly, particularly in the areas of lens polishing, lens grinding, and complex lens train assembly. Precision Machining Expertise: Excellence with Every Component The Norwalk facility boasts a wide array of precision machining capabilities, each designed to meet the exacting standards of the medical industry. Swiss Style CNC Lathes: Excel in turning and milling intricate parts with exceptional precision. 5 Axis Machining Centers: Unparalleled versatility, creating complex geometries with ease. CNC Milling: High precision milling for intricate part production. Surface Finishing: Ensuring every component meets…