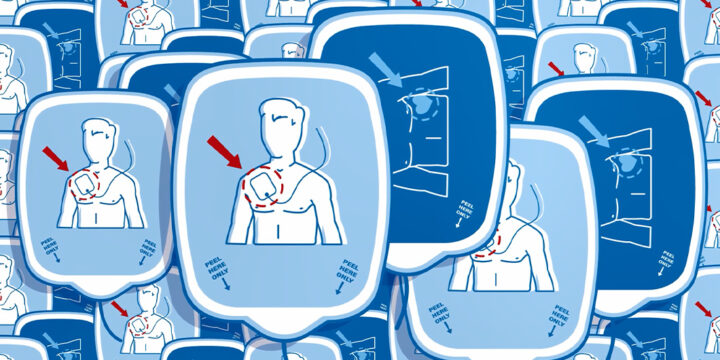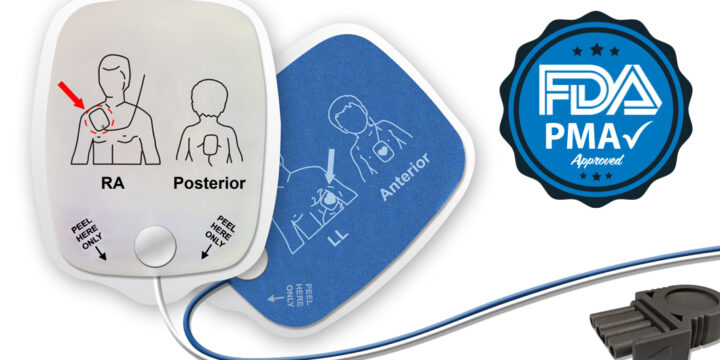
PMA Meaning in Medical Devices: HeartSync’s FDA Approval
What is the PMA meaning in medical manufacturing, and why does it matter? PMA stands for Premarket Approval, the FDA’s most rigorous pathway for approving high-risk medical devices. In July 2023, Nissha Medical Technologies (NMT) received PMA approval for its HeartSync line of disposable defibrillation electrodes. This marked a milestone that reflects years of collaboration, testing, and innovation.PMA Meaning vs. 510(k): What's the Difference?To understand the definition of PMA, it helps to compare it to the more familiar 510(k) clearance process.The 510(k) pathway applies to moderate-risk devices. It allows manufacturers to demonstrate that their product is substantially equivalent to one already on the market.PMA approval is required for Class III, or high-risk, devices. Therefore, these products require extensive testing, clinical data, and a full FDA review before they can be…