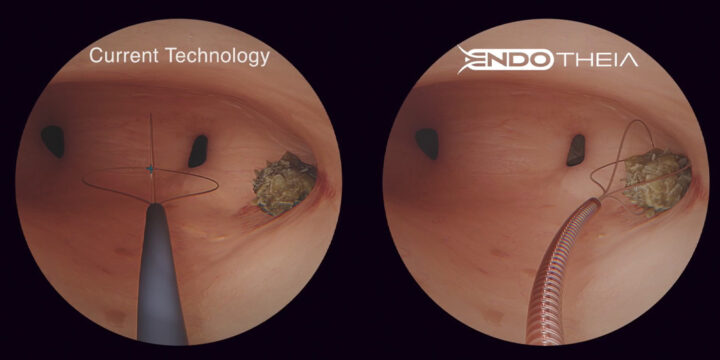
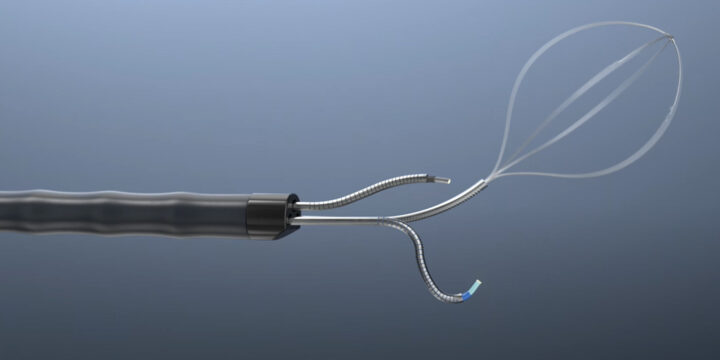
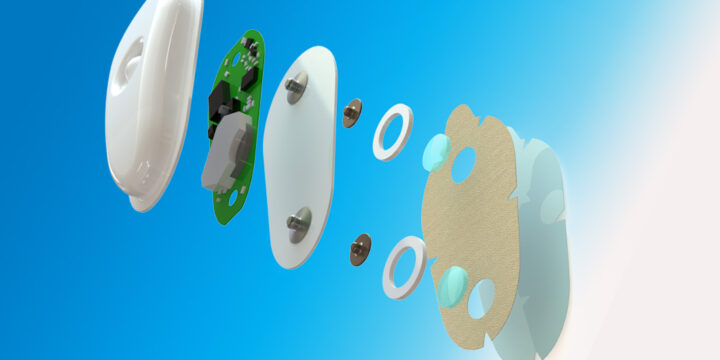
The Shift Toward Nearshoring in Medical Device Manufacturing
By Amy JearyGlobal supply chain disruptions and evolving trade policies, including potential new tariffs, are prompting medical device manufacturers to rethink sourcing strategies. As geopolitical uncertainties and regulatory shifts loom, companies are increasingly turning to nearshoring—moving production closer to home—to reduce lead times, improve resilience, and navigate potential cost increases more effectively.Why Medical Device Manufacturers Are Nearshoring1. Strengthening Supply ChainsThe COVID-19 pandemic exposed vulnerabilities in global supply chains, with shipping delays and material shortages disrupting production. Nearshoring shortens supply chains, reduces reliance on global logistics, and enables faster market responses.2. Faster Lead TimesNearshoring significantly reduces shipping times, enabling companies to react quickly to demand fluctuations, regulatory changes, and urgent medical needs—critical for high-demand devices like diagnostic equipment and surgical instruments.3. Cost and Regulatory BenefitsAlthough Far East labor costs are lower,…