Latest blogs
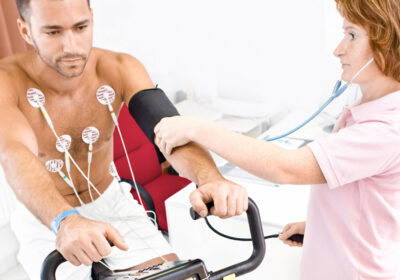
What Makes Stress Test Electrodes Different?
Choosing the right electrode is critical to capturing accurate ECG signals. In high-motion testing scenarios like stress tests, high-quality electrodes help eliminate signal noise, reduce the risk of repeat procedures, and support confident clinical decisions.
The Silent Killer – How ECGs Detect Hidden Heart Attacks
Nearly half of heart attacks strike silently, causing heart damage without noticeable symptoms. This type of case doesn’t just affect the older generation. Even young, healthy individuals are at risk of silent, but deadly cardiovascular issues. 1 in 5 heart attack patients are under 40, and cases such as these are rising. Cardiovascular monitoring is essential for catching cardiovascular problems before they arise. The developing use of AI is revolutionizing electrocardiogram (ECG) analysis, providing early detection and life-saving interventions before it’s too late.
What are Hypoallergenic ECG Electrodes? How to Accommodate Sensitive or Fragile Skin
The most important aspect when finding the best electrode for you or your company is knowing the needs of your patients. By considering each of the different components that make up your EKG electrode you can pinpoint which factors are most beneficial to your needs.
About Us
NMT 360° is your resource for up to date, interesting information about products, industry news, instructions for use and much more. With our teams cumulative years of experience, from marketing, to engineering to quality, we’re certain there will be something for everybody.